About Us
About Us
We are Engineering & Consultancy Company promoted to cater the requirement of Facility Design and providing Pharma Process Equipment and Utilities, Turnkey Solutions for Heating Ventilation Air Conditioning (Automated HVAC), Modular -Cleanroom, IBMS, Metal (Fire) Doors , Electrical & Fire Fighting Project for Healthcare, Pharmaceuticals. Micro-Electronics, Food-Processing & Bio-Technology.
We have extensive and vast experience in delivering success in engineering projects including Consulting, Design, Supplies, Installation, Testing, Commissioning, Validation for the Facilities Systems with documentation that meets up to GMP /WHO/ NABH / UK MHRA / US FDA and others.
What we Offer
Bio Clean offers a complete one stop project solution and can supply a variety of experienced professionals from technical writers and engineers all the way to project managers.
We execute cleanroom projects spotlessly within shortest possible time frame coupled with our unique project management philosophy which would live out through the cycle of the project as each product & service is tailored made to client’s necessities.
Bio Clean provides key services to align with effective GMP framework and Pharmaceutical involved Project Management. Bio Clean look at client’s requirement from the perspective of feasibility and result. The services that are required to bring an idea to a working facility producing drugs that was envisioned at the beginning has always been a challenge and we have been steering through these challenges all these years where all our services under one roof provide customers an ease to find answers to all its needs.

Our Engineering Process
The Six Stages of a Bio Clean Engineering and Design process
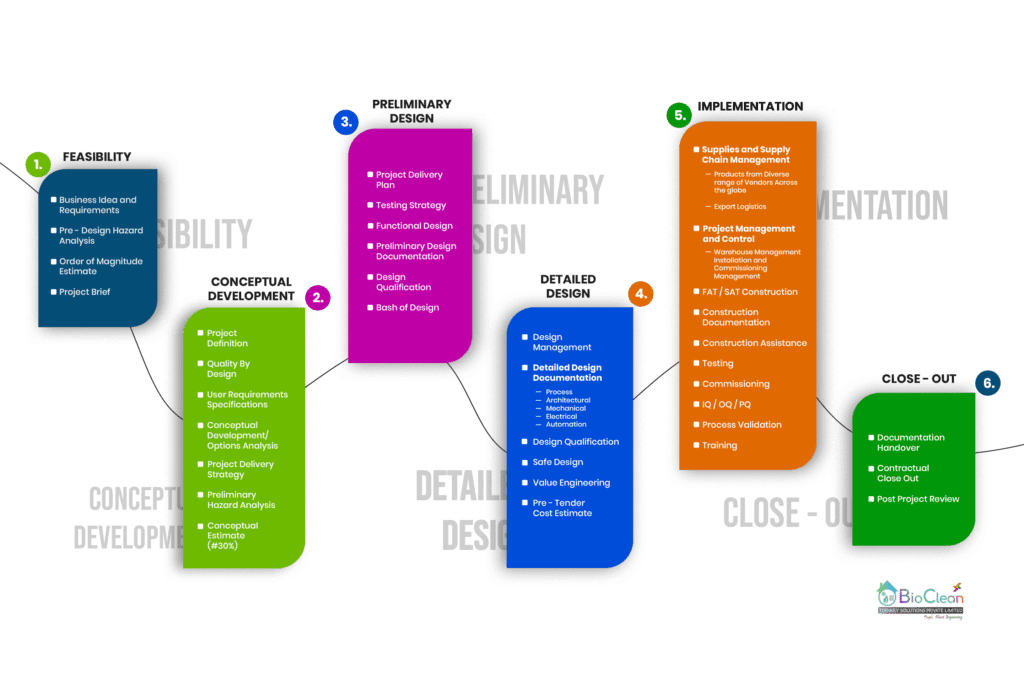
*The Feasibility and Conceptual development stages are often combined and performed to make ensure the project makes sense once all factors are considered and the project has a potential viable business case with scope of project defined & estimated cost is determined.
*Facility Buildings are often built first and then the process shoe-horned into the available space. This creates workflow and regulatory compliance issues. Our Process Engineers ensure they understand the process first before designing the facility. Our approach is based on ‘Quality By Design’ (QbD) principles and uses any laboratory scale up. We also ensure there is a “loose fit”, permitting future expansion and the inclusion of other operations later on.







