Engineering Consultancy
Engineering Consultancy
We offer Facility Planning, Engineering Design and Project Management services to the Pharmaceutical, Hormones, Vaccines, Biotechnology, Veterinary, Micro-Electronics and Medical Devices industries.
It requires a clear understanding of not only the process but also requires consideration of cGMP regulations, regulatory compliance, local statutory norms, quality management and special care for cross contamination, gowning, EHS, cleaning, air flow control and water purification. Bio Clean’s GMP consulting services are provided by our international Good Manufacturing Practice cGMP consultants and experts. Many in our team have previously completed successful projects with consultation in Bangladesh, Oman, India, Senegal, Uganda etc.
We are fully capable of designing facilities that are fully compliant with USFDA, EMEA, WHO, MHRA, PIC or any other global / local regulatory requirements.
Our Engineering Process
The Six Stages of a Bio Clean Engineering and Design process
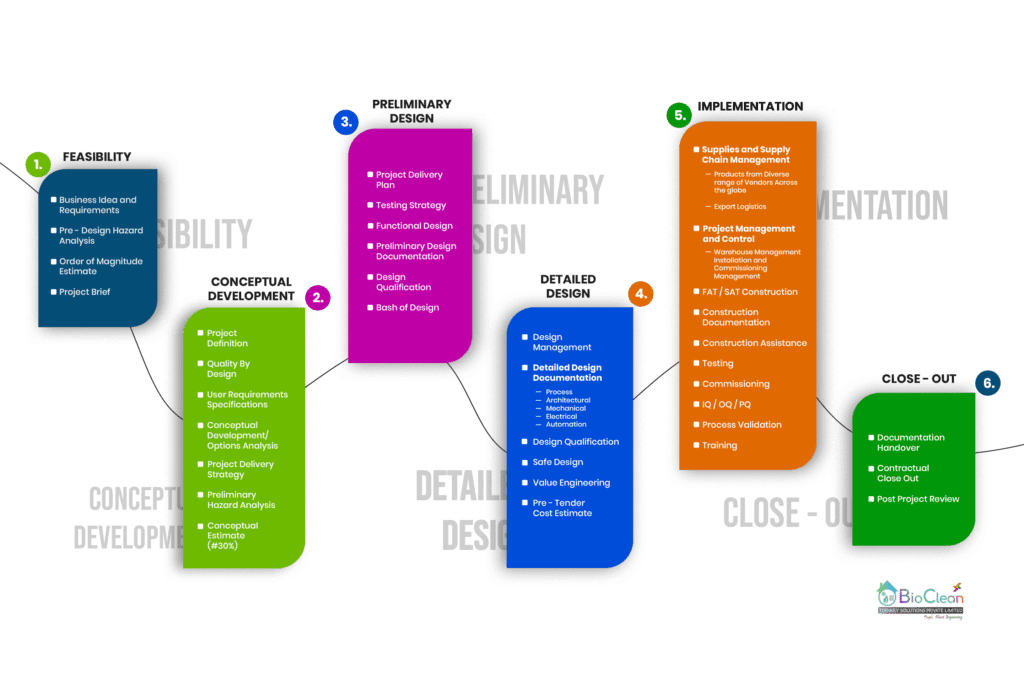
From our Engineering Process Flow, Engineering Consultancy consist of 4 Stages,
Feasibility Studies are performed to make ensure:
the project makes sense once all factors are considered
the estimated cost is determined (±30%)
there are enough resources available to meet a client’s requirements for the facility.
the project has a potentially viable business case
information is provided about potential design options
the scope of the project is defined
then Client can decide whether to proceed with the product, After the feasibility study we do an Initial Engineering Concept Design, starting with your product and market requirements, documented in a User Requirements Specification.
Our Process Engineers are practiced in equipment selection, the advantages and disadvantages of certain technologies and, based on our industry experience, we can therefore advise on the best options. We will work closely with your team to develop the most appropriate facility design based on your process requirements while also achieving GMP compliance. Our complete GMP compliance, engineering and validation service will ensure a high level of confidence that the correct GMP target / standard is set.
Related Services

TURNKEY CLEANROOM SOLUTIONS
WALKABLE CEILING PANELS
FIRE RATED DOORS
METAL DOORS

ELECTRICALS & CONTROLS
HVAC CONTROLLERS AND SENSORS
BUILDING MANAGEMENT SYSTEM
DOOR INTERLOCKING SYSTEM
DDC - HMI & PLC - SCADA
TURNKEY HVAC SOLUTIONS
PRE FABRICATED DUCTS
AIR HANDLING UNITS
DEHUMIDIFIERS

PHARMA UTILITIES & DISTRIBUTION
COMPRESSED AIR
STEAM ETC

PHARMA PROCESS EQUIPMENT
TRACK AND TRACE SYSTEM
PACKING EQUIPMENT

FIRE FIGHTING SYSTEM
GAS SUPPRESSION SYSTEM
FIRE ALARM SYSTEM
FIRE EXTINGUISHER







