PARENTERAL Products
Parenteral dosage forms differ from all other drug dosage forms, because they are injected directly into body tissue through the primary protective systems of the human body, the skin, and mucous membranes. They must be exceptionally pure and free from physical, chemical, and biological contaminants. These requirements place a heavy responsibility on the pharmaceutical industry to practice current good manufacturing practices (cGMPs) in the manufacture of parenteral dosage forms and on pharmacists and other health care professionals to practice good aseptic practices (GAPs) in dispensing parenteral dosage forms for administration to patients.
Certain pharmaceutical agents, particularly peptides, proteins, and many chemotherapeutic agents, can only be given parenterally, because they are inactivated in the gastrointestinal tract when given by mouth. Parenterally-administered drugs are relatively unstable and generally highly potent drugs that require strict control of administration to the patient. Due to the advent of biotechnology, parenteral products have grown in number and usage around the world.
Characteristics of parenteral dosage forms
• Parenteral products are unique from any other type of pharmaceutical dosage form for the following reasons:
• All products must be sterile.
• All products must be free from pyrogenic (endotoxin) contamination.
• Injectable solutions must be free from visible particulate matter.
This includes reconstituted sterile powders.
• Products should be isotonic, although strictness of isotonicity depends on the route of administration.
• Products administered into the cerebrospinal fluid must be isotonic.
• Ophthalmic products, although not parenteral, must also be isotonic. Products to be administered by bolus injection by routes other than intravenous (IV) should be isotonic, or at least very close to isotonicity.
• IV infusions must be isotonic.
• All products must be stable, not only chemically and physically like all other dosage forms, but also ‘stable’ microbiologically (i.e., sterility, freedom from pyrogenic and visible particulate contamination must be maintained throughout the shelf life of the product).
• Products must be compatible, if applicable, with IV diluents, delivery systems, and other drug products co-administered.
CLASSIFICATION Injections may be classified in six general categories:
• 1. Solutions ready for injection
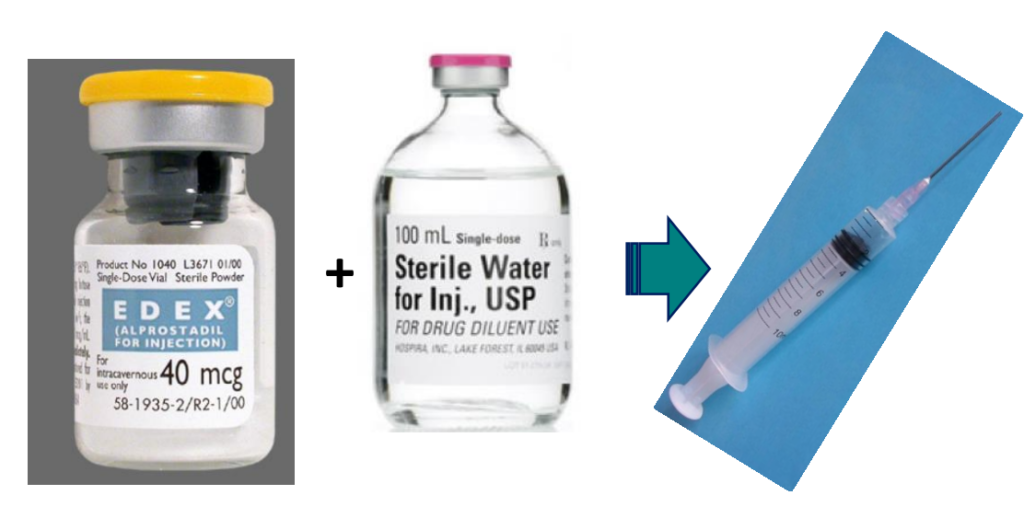
• 2. Dry, soluble products ready to be combined with a solvent just prior to use
• 3. Suspensions ready for injection
• 4. Dry, insoluble products ready to be combined with a vehicle just prior to use
• 5. Emulsions
• 6. Liquid concentrates ready for dilution prior to administration Components
• Components of parenteral products include the active ingredient, formulation additives, vehicle(s), and primary container and closure. Establishing specifications to ensure the quality of each of these components of an injection is essential. Secondary packaging is relevant more to marketing considerations, although some drug products might rely on secondary packaging for stability considerations, such as added protection from light exposure for light sensitive drugs and antimicrobial preservatives.
PREPARATION
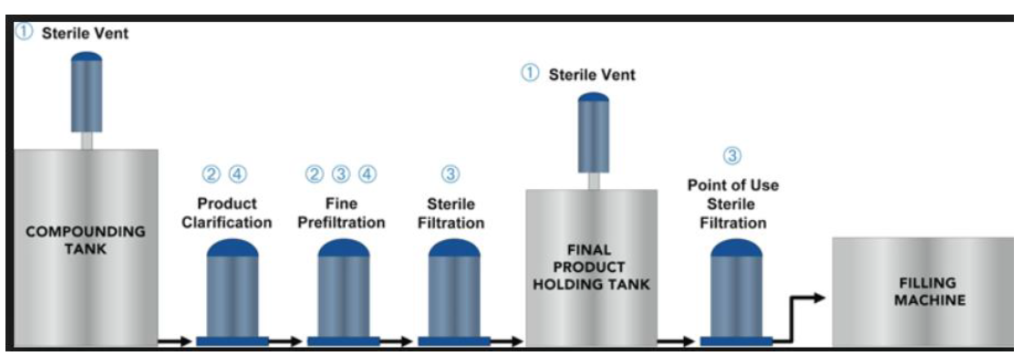
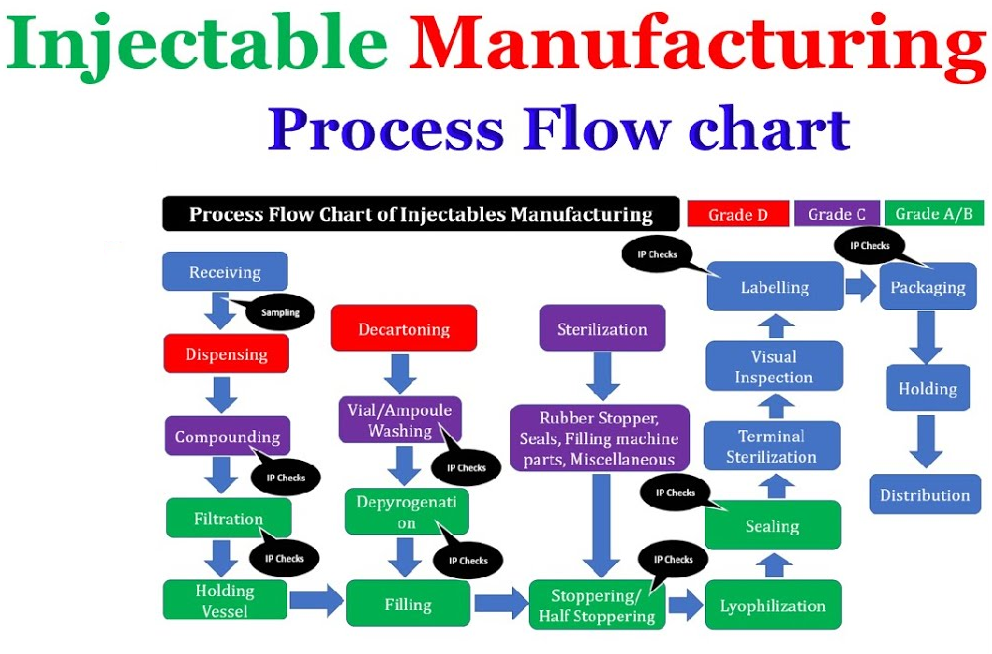
• Primary washing and sterilization
• Compounding
• Terminal sterilization
CONTROL/TESTS – Particulate contamination – Sterility – Bacterial endotoxins and pyrogens – Uniformity of dosage units – Uniformity of content – Uniformity of mass
Containers and closures
• Injectable formulations are packaged into containers made of glass or plastic. Container systems include ampoules, vials, syringes, cartridges, bottles, and bags
Containers and closures
• Ampoules
• Glass vials sealed with rubber stoppers
• Plastic ampoules (blow-fill-seal)
• Pre-filled syringes
• Needle-free injection Small volume parenterals (SVPs)
• Ampoules – heat sealed after filling
• Glass vials sealed with rubber stoppers
• Plastic ampoules (blow-fill-seal)
• Pre-filled syringes – reducing the degree of manipulation required – facilitating administration in an emergency situation
• Needle-free injection
Large volume parenterals (LVPs)
• Glass bottles sealed with rubber stoppers
• Plastic bags