Oral Solid dosages
Oral solid dosage (OSD) products can take several different shapes, and with those different forms comes different production techniques and facility designs.
Small molecules, tablets, capsules, soft gels, effervescence, gummies, and pills. These are all oral solid dosage (OSD) forms, a term that refers to a final drug product therapy that is ingested through the mouth, dissolved in the digestive system, and delivered to the body through absorption into the bloodstream. oral solid dosage drug products are the most common dose form physicians prescribe for a variety of indications.
Oral solid dosage is such a dominant delivery form for three main reasons. It’s relatively easy to administer, it’s easy to distinguish one oral solid dosage product from another, and oral solid dosage manufacturing methods are well understood and well-developed.
The most common oral solid dosage forms are tablets and capsules. Both forms are comprised of an active pharmaceutical ingredient (API), which can also be called a drug substance, and dry powder ingredients. Tablet forms are made through compression and can either be coated, meaning they have an extra layer to create a smooth surface, or uncoated. Capsules are created through a coating process, where the drug substance and dry ingredients layer around a seed material.
Each of these forms can have variable bioavailability and rate of release. Depending on the therapeutic use of the OSD product, it will have an immediate, sustained, controlled, or extended-release. These factors influence drug manufacturing platforms and the equipment and technology used in the manufacturing process.
The overall goal for OSD processing regardless of the type of product is to create a formulation that ensures each dose–a single tablet or capsule–is consistent. Each one has a repeatable distribution of ingredients, and there is a consistency of dissolution and bioavailability to ensure that the drug product is safe and effective
PRIMARY OSD MANUFACTURING UNIT OPERATIONS
One of the unique aspects of oral solid dosage manufacturing is that the typical unit operations (steps in the process) are very well defined and relatively unchanged over the past century. Although the unit operations may involve various equipment and technologies, there is a well-defined progression from raw materials into the final product.
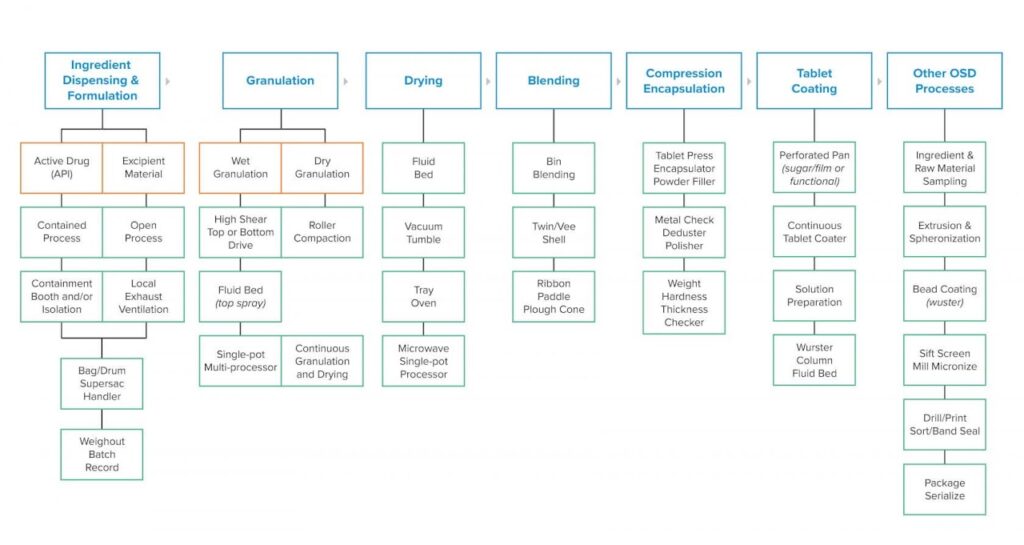
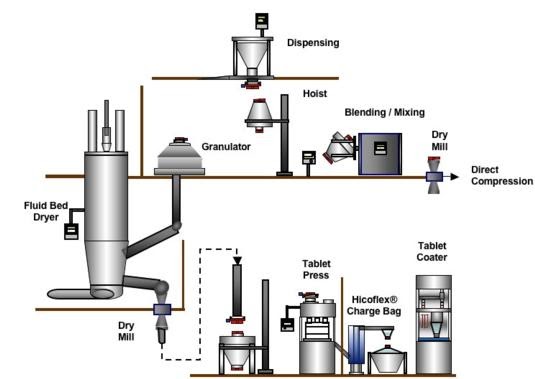
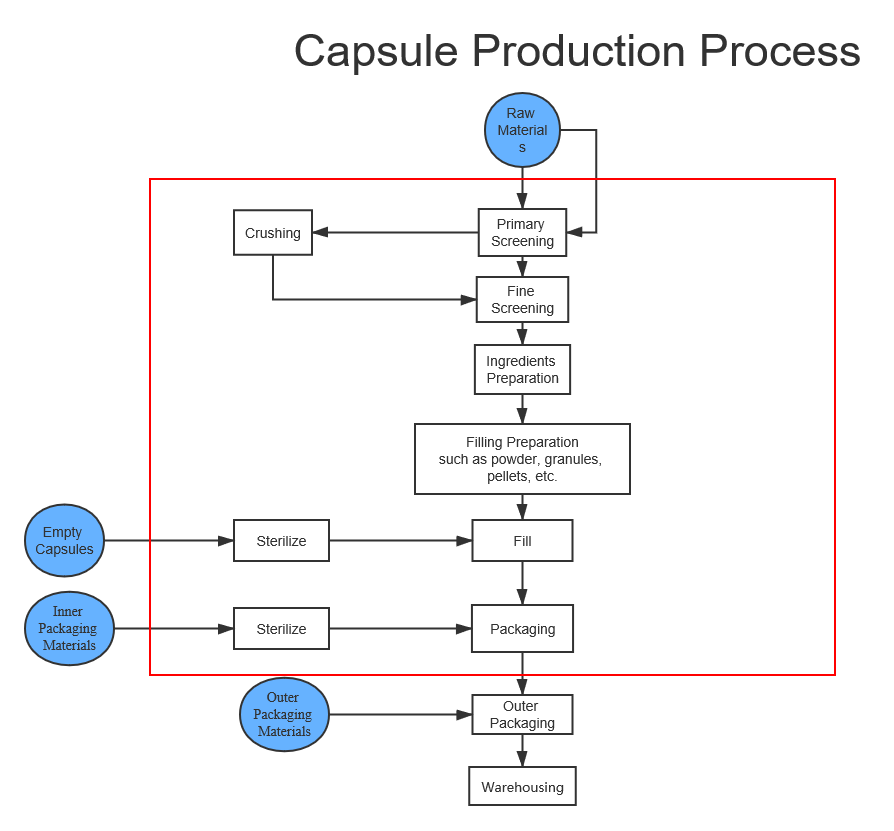
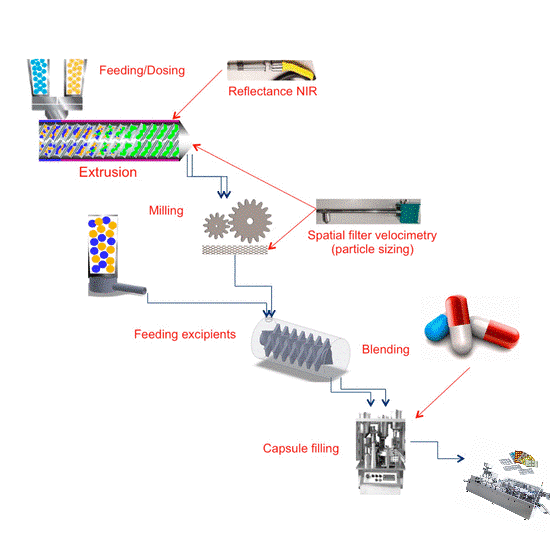
This matrix illustrates the basic production flow from left to right. The boxes along the top represent the unit operation activity. This can vary with specialized drug products and unique applications, but this flow is the norm.
Oral solid dosage (OSD)- Capsule.
Capsules are solid pharmaceutical dosage forms in which the drug or a mixture of drugs is enclosed in a Gelatin Shell or any other suitable material to form various shapes. Capsules generally contain a single dose of active ingredients and are taken orally.
Excipients such as opaque fillers, antimicrobial preservatives , sweetening agents, permitted flavoring agents and one or more coloring agents maybe added. Capsules may be printed on the outer surface.
Capsules may contain medications in solid, paste or liquid forms. The API filled in the capsules may contain excipients as solvents or fillers but the shell should not be attacked by the content thereby releasing the contents of the capsule.
The contents of capsules other than Modified-release (Sustained-release) do not contain any added coloring agent.
There are following types capsules:
1. Hard Gelatin Capsules
2. Soft Gelatin Capsules
3. Modified Release Capsules
4. Enteric Capsules
1. Hard Gelatin Capsules:
Hard gelatin capsules contain the active ingredients in the form solids. When two drugs of different properties are added in a capsule, one of them should be in the form of the tablet, pellet or small capsule and then enclosed in the large capsule.
Hard gelatin capsules are manufactured by dipping capsule shell shaped pins into liquid gelatin solutions, after that gelatin films on the pins are dried, trimmed, and then removed from the pins. Body and cap pieces are supplied unlocked.
2. Soft Gelatin Capsules:
Soft gelatin capsules made from gelatin (sometimes called soft gel) are somewhat thicker than that of hard gelatin capsules and may be plasticized by adding the sorbitol or glycerin. The ratio of plasticizer depends upon the environmental conditions as well as the nature of the contents filled into the soft gelatin capsule.
Like hard gelatin shells, the composition may include approved dyes and pigments and preservatives. Flavors may be added and up to 5% sucrose may be included for its sweetness and to produce a chewable shell. Soft gelatin capsule shell generally contains 6% to 13% of water.
Soft gelatin capsules are generally filled during their formation and sealed with machines.
Soft gelatin capsules generally contain liquid or solid drugs dissolved in suitable liquids to form a paste. In some cases, granules and powders maybe filled with the soft gelatin capsules.
3. Modified Release Capsules:
Modified-release capsules are hard or soft gelatin capsules in which the contents or the shell, or both are prepared by a special process to modify the rate of releasing the active ingredients of the capsule.
4. Enteric Capsules:
Enteric Capsules are hard or soft gelatin capsules those are resisted against the gastric fluid but dissolves in the intestinal fluid to release its contents.
During manufacturing, packaging, storage and distribution of capsules, their microbial quality should be maintained as per their acceptance criteria because of the possibilities of microbial growth in gelatin.