Oral Liquid
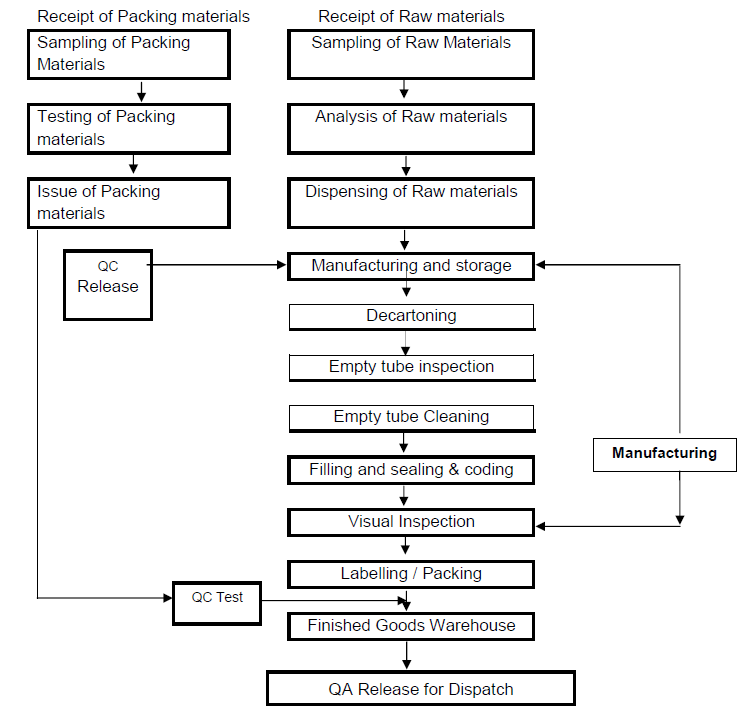
Oral Liquid Dosage Forms in Pharmaceuticals including Syrup, Oral Suspension, Oral Solution, Oral Drop, Oral Emulsion, Mixture, Linctuse and Elixir. Oral liquids are homogeneous liquid preparations, usually contains a solution, an emulsion or a suspension of one or more active ingredients in a suitable liquid base. They are prepared for oral administration either as such or after dilution. They may contain other substances such as suitable dispersing, solubilizing, wetting, emulsifying, stabilizing, suspending, thickening agents and antimicrobial substances for preservation. They may also contain suitable sweetening agents, flavoring agents and permitted coloring agents. If sodium saccharin or potassium saccharin is used for sweetening, then its concentration in pediatric preparations should not be more than 5 mg per kg of body weight. During manufacturing, packaging, storage and distribution process of oral liquids, microbial quality should be maintained and microbial count should be within the acceptance criteria. Oral Liquids should not be diluted and stored after dilution unless the individual monograph directs for dilution. Diluted oral liquids may not be stable for long period physically and chemically so it should be diluted freshly or should be used within the period as stated on the label.
Types of oral liquids:
1. Syrup
2. Oral Suspension
3. Oral Solution
4. Oral Drop
5. Oral Emulsion
6. Mixture
7. Linctus
8. Elixir
Drug solubility In pharmaceutical solutions both the therapeutic agent and the excipients are legally required to be present in solution over the shelf-life of the formulated product. As a result pharmaceutical solutions are termed homogeneous. One of the major challenges to the pharmaceutical scientist is the attainment of homogeneity in the formulation, due primarily to, in many cases, the limited aqueous solubility of the therapeutic agent.
Initially there are possible scenarios regarding the formulation of pharmaceutical solutions of a therapeutic agent for oral administration:
■ The aqueous solubility of the therapeutic agent is high at the selected pH of the formulation. Under these circumstances the therapeutic agent may be readily incorporated into the vehicle and formulated as an oral solution.
■ The aqueous solubility of the therapeutic agent is moderate at the selected pH of the formulation, i.e. the aqueous solubility is less than the requested concentration of therapeutic agent.
■ The aqueous solubility of the therapeutic agent is low at the selected pH of the formulation.
The difference between the aqueous solubility of the therapeutic agent and the required concentration is too great to be bridged by the use of co-solvents and related methods or the concentration of co solvents or surfactants in the solubilized formulation may be toxic when administered orally. The drug may therefore be formulated as an alternative-dosage form, e.g. a suspension. Prior to discussing the solubility of therapeutic agents and formulation strategies to modify this property, it is worth considering the process of drug dissolution.